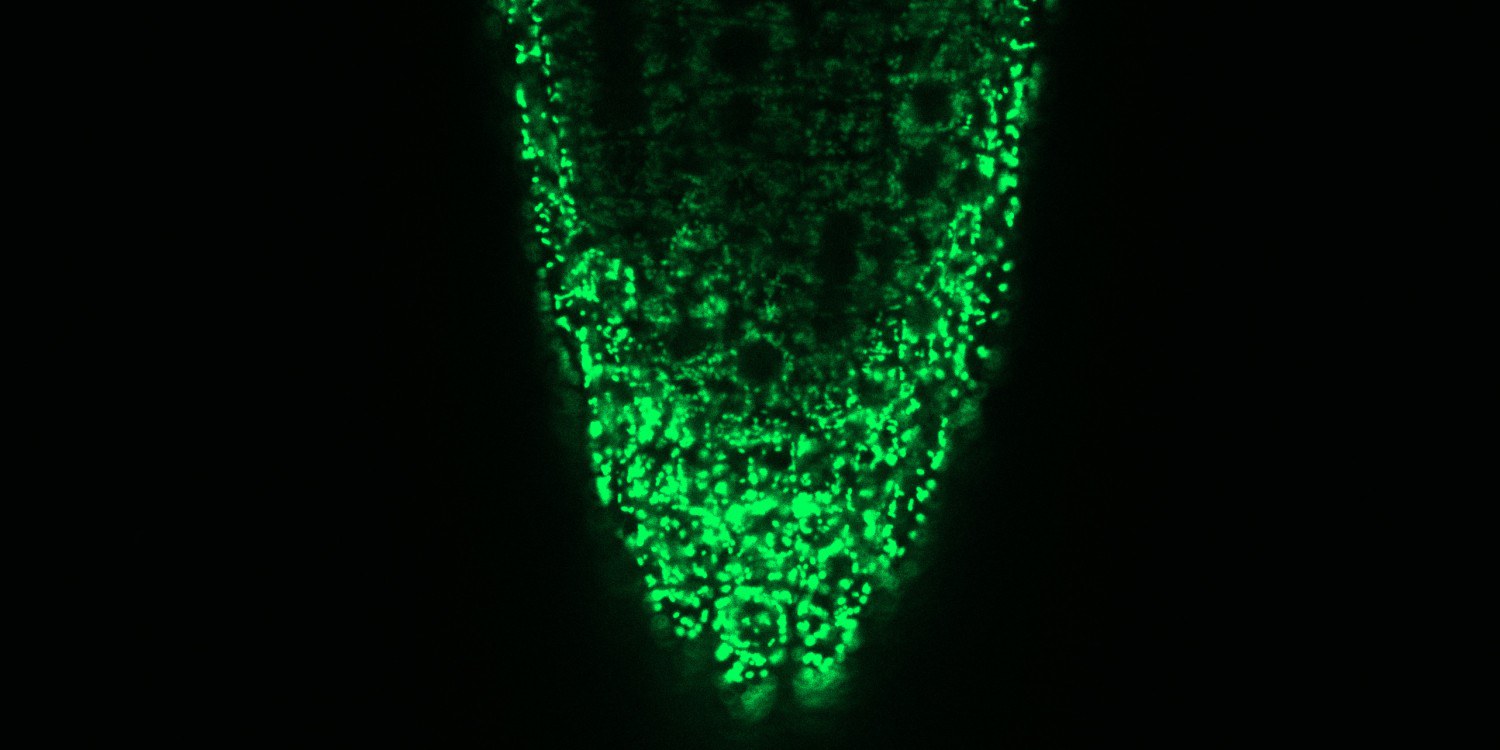
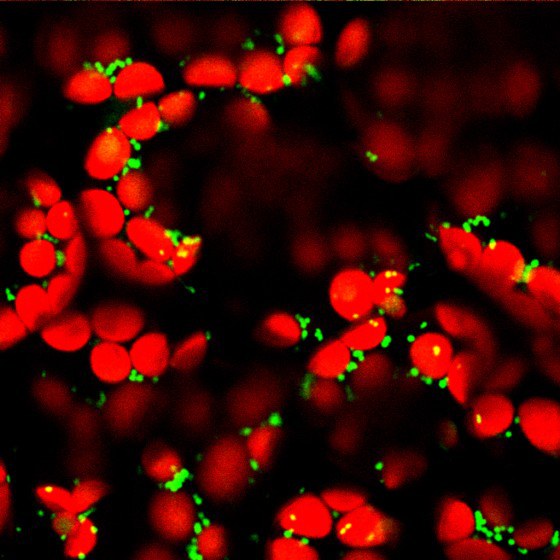
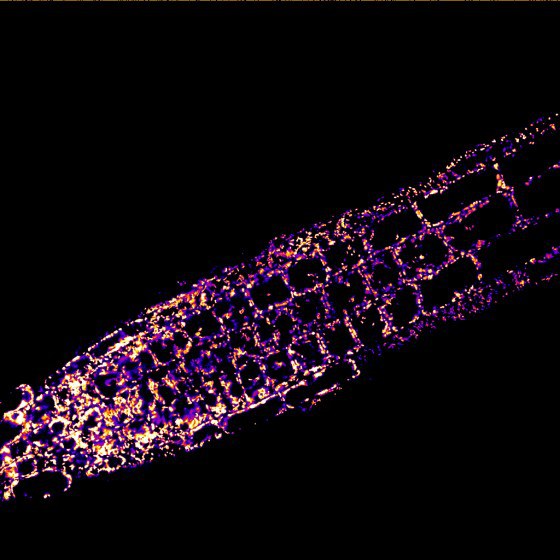
“It is astonishing that such a simple ion can be so important for transmitting information,” says Markus Schwarzländer. “We assume that the calcium ions develop this potential through the exact place and the time of their deployment.” It has already been known since 1965 that plant mitochondria can take up calcium ions and in this way – presumably – be involved in calcium signalling pathways. How exactly the transport is made possible was, however, disputed for decades. For most ions, the inner mitochondria membrane is impermeable, but certain proteins in the membrane can ensure that the calcium ions can pass through this partially permeable membrane and thus enable signals to be transmitted in this cell organelle.
In the case of animals, the question of the identity of the mitochondrial calcium channel was solved in 2011 when researchers at the Universities of Harvard and Padua discovered the calcium channel MCU (mitochondrial calcium uniporter). This breakthrough paved the way for the finding that plants also contain MCU genes. What was still unclear, however, was whether these genes also form calcium channels in the living cell – not least because the uptake of calcium ions into animal mitochondria displays markedly different patterns from those into plant mitochondria.
Gene expression reveals importance of calcium ion transport for cellular power stations
In order to clarify the role played by MCUs in plant cells, the Münster researchers had to simultaneously deactivate three of the six MCU genes in the model plant Arabidopsis thaliana. As a result, they restricted the capacity of the cellular machinery and were thus able, for the first time, to observe in a living plant the consequences which this constraint leads to. For this purpose, they used a fluorescent protein which indicates changes in the concentration of calcium ions in the mitochondria in the form of a light signal. What could be seen was that, as a result of the MCU genes having been deactivated, a much lower number of calcium ions got into the mitochondria. This means that the researchers have demonstrated not only that living plant cells – similarly to animal cells – transport their calcium ions into the mitochondria through the MCU channels. They were also able to show that this is by far the most important path of rapidly transporting calcium ions into the mitochondria.
After this observation, the team used plants with impaired mitochondrial calcium transport capacity to try to find out what role mitochondrial calcium plays for the plant and its fitness. In the case of animals, calcium ions in the mitochondria regulate energy production – but there were no indications of a similar function in plants.
By analysing the expression of the entire plant genome, the researchers were now able to demonstrate that the reduced transport capacity for calcium ions has an impact on the regulation of the plant hormone jasmonic acid. Jasmonic acid is a defence hormone in plants which provides protection against herbivores by being activated if the plant is wounded. Amongst other things, jasmonic acid also controls senescence – i.e. the regulated dying-off of tissues – as well as responses to mechanical stimuli such as being touched. The plants manipulated by the researchers showed slightly delayed senescence: in dark surroundings, the leaves lost their green pigmentation less rapidly. They also displayed a markedly weaker response to touch. “What is particularly surprising for us,” says Schwarzländer, “is that there is evidently a link between the transport of calcium ions into the mitochondria and the regulatory process steered by the jasmonic acid. The results show that molecular processes such as the absorption of calcium ions in the mitochondria, which have been conserved in animals and plants through evolution, can be used to serve new functions.”
One of the central methods used in the study now published was “in vivo biosensorics”. Here, proteins are designed – using molecular-biological and biotechnological methods – in such a way that they serve as synthetic measuring sensors in living organisms.
The working groups led by Markus Schwarzländer and Alex Costa constitute, in equal parts, the core of the study. Lead author Dr. Cristina Ruberti had first been researching in Münster for two years before she moved to the Università degli Studi in Milan. The partners included teams from Halle, Heidelberg, Bonn, Lund (Sweden), Antwerp (Belgium) and Viçosa (Brazil).
Publication: Cristina Ruberti, Alex Costa, Markus Schwarzländer et. al.: MCU proteins dominate in vivo mitochondrial Ca2+ uptake in Arabidopsis roots. The Plant Cell (2022); DOI: 10.1093/plcell/koac2421
To the press release of the University of Münster:
www.uni-muenster.de | 08.08.20222